What signaling pathways are activated in lipolytic adipocytes?
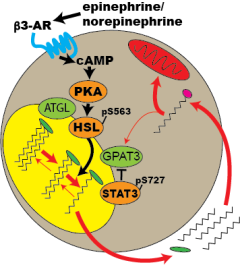
Catecholamine stimulation activates white adipocyte lipolysis, leading to the release of free fatty acids from the lipid droplet. A portion of these free fatty acids released are taken back up by the adipocyte where they may be re-esterified and stored or oxidized. Understanding the signaling pathways that activated during adipocyte lipolysis will help gain valuable insight into the fate of these fatty acids.
What kinase phosphorylates STAT3 at serine 727?
Following catecholamine stimulated of adipocyte lipolysis, free fatty acids are released from the lipid droplet. A fraction of these fatty acids are retained within the adipocyte and activate a signaling cascade that results in the phosphorylation of STAT3 at serine 727. We seek to investigate the kinase responsible for this phosphorylation event.
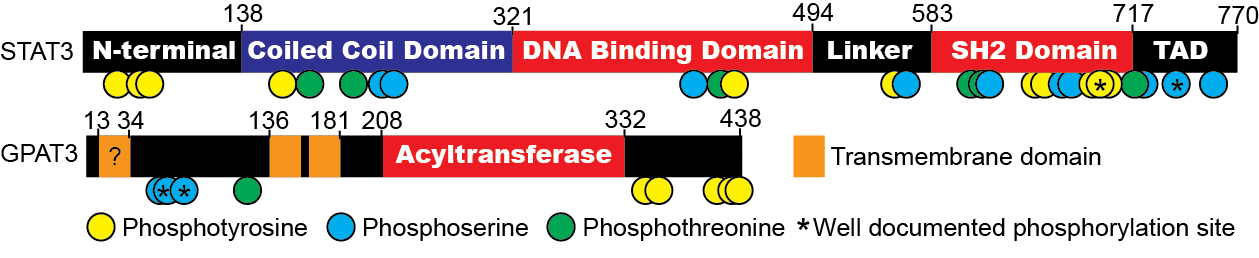
Identification of the interaction domains of STAT3 and GPAT3
STAT3 co-immunoprecipitates (co-IPs) with GPAT3 and reciprocally, GPAT3 co-IPs with STAT3. To map the domain(s) of STAT3 that are necessary and sufficient to interact with GPAT3, we are performing co-IP assays with a series of deletion mutants. We hypothesize that the C-terminal STAT3 transcription activation domain (TAD) will be critical for interaction with GPAT3 (Fig. 9), as our preliminary data indicate that phosphorylation of Ser727 residue in this domain enhances this interaction. We also created N-terminal and C-terminal GPAT3 deletion mutants both with and without the hairpin membrane anchor. These constructs will be used in co-IP assays with STAT3 to determine which terminus interacts with STAT3 and whether or not anchorage to the membrane impacts this interaction.